PDF) Compliance with requirement to report results on the EU Clinical Trials Register: Cohort study and web resource
What Are the Documents Required for Clinical Trial Applications to Regulatory Authorities in Europe? - Sofpromed

Clinical trial success relies on effective patient recruitment – International Clinical Trials Day 2022 | ECRIN

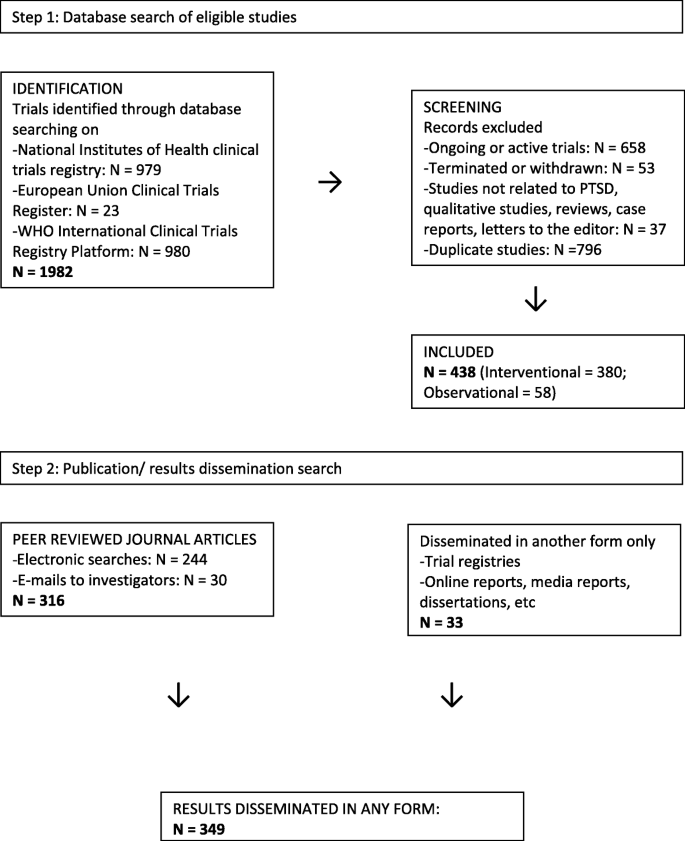
Flow of diagram of study. Abbreviation: eU-cTr, european Union clinical... | Download Scientific Diagram

Prevalence of clinical trial status discrepancies: A cross-sectional study of 10,492 trials registered on both ClinicalTrials.gov and the European Union Clinical Trials Register. | Bennett Institute for Applied Data Science
Publication and non-publication of clinical trials in PTSD: an overview | Research Integrity and Peer Review | Full Text

Research Techniques Made Simple: Workflow for Searching Databases to Reduce Evidence Selection Bias in Systematic Reviews - ScienceDirect

Flow of diagram of study. Abbreviation: eU-cTr, european Union clinical... | Download Scientific Diagram