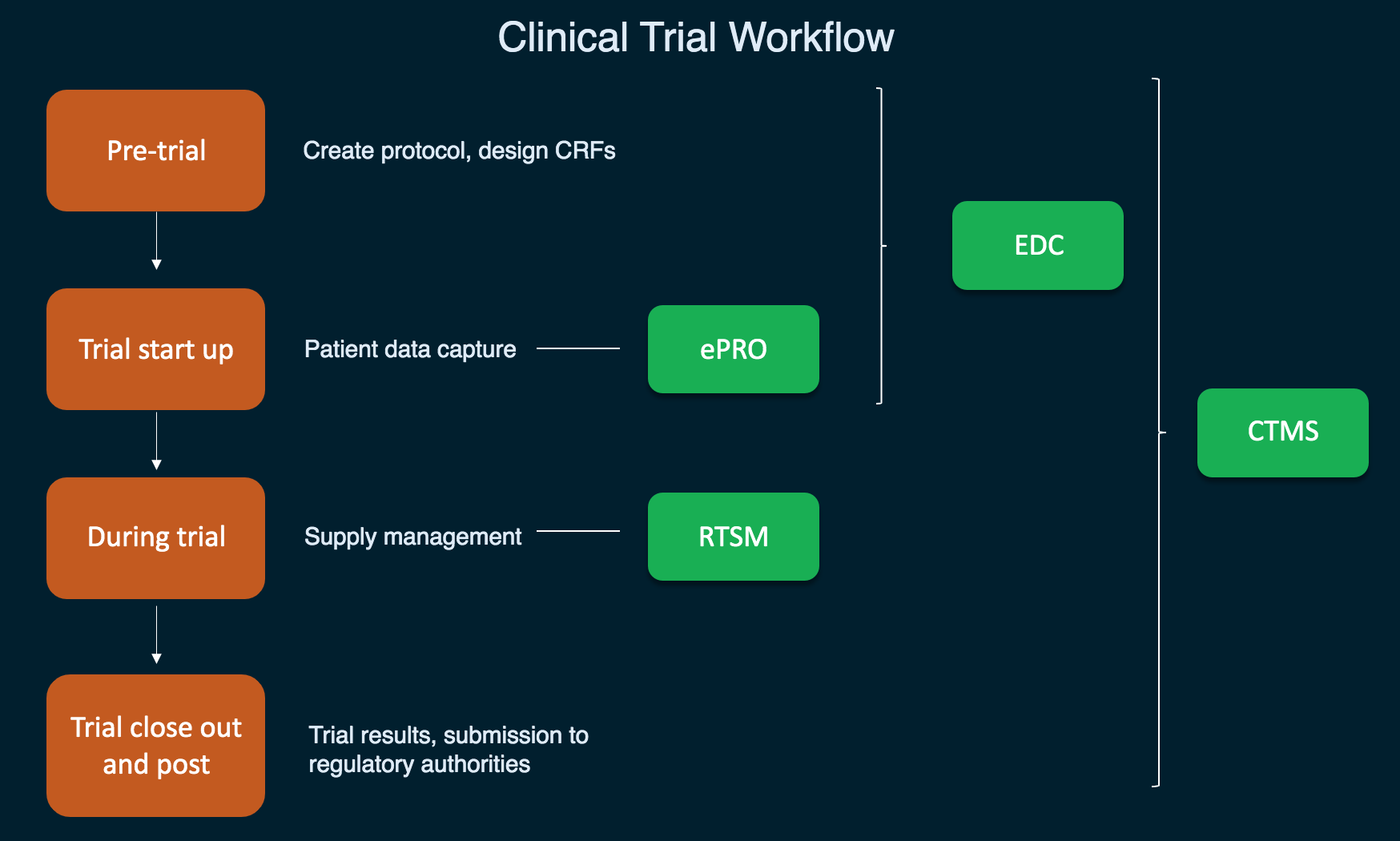
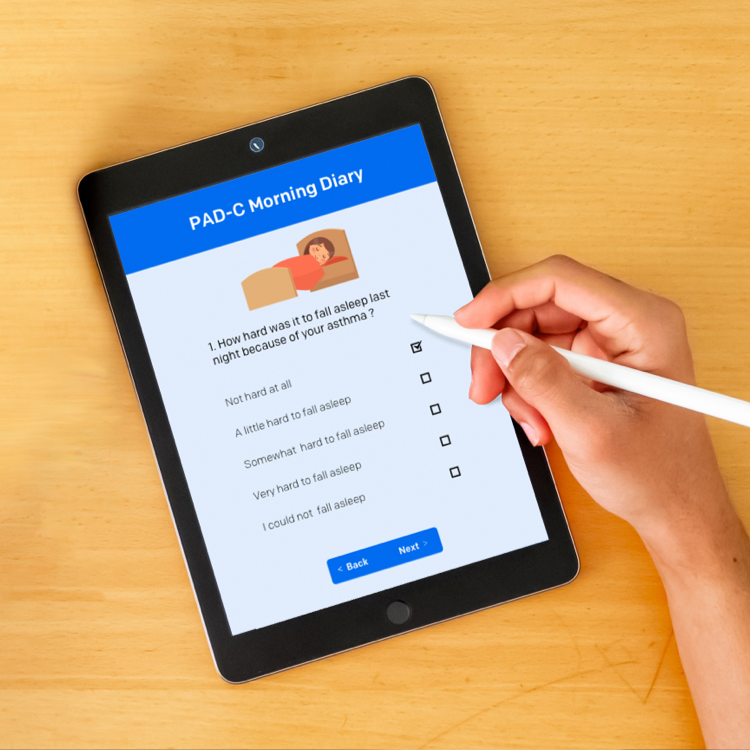
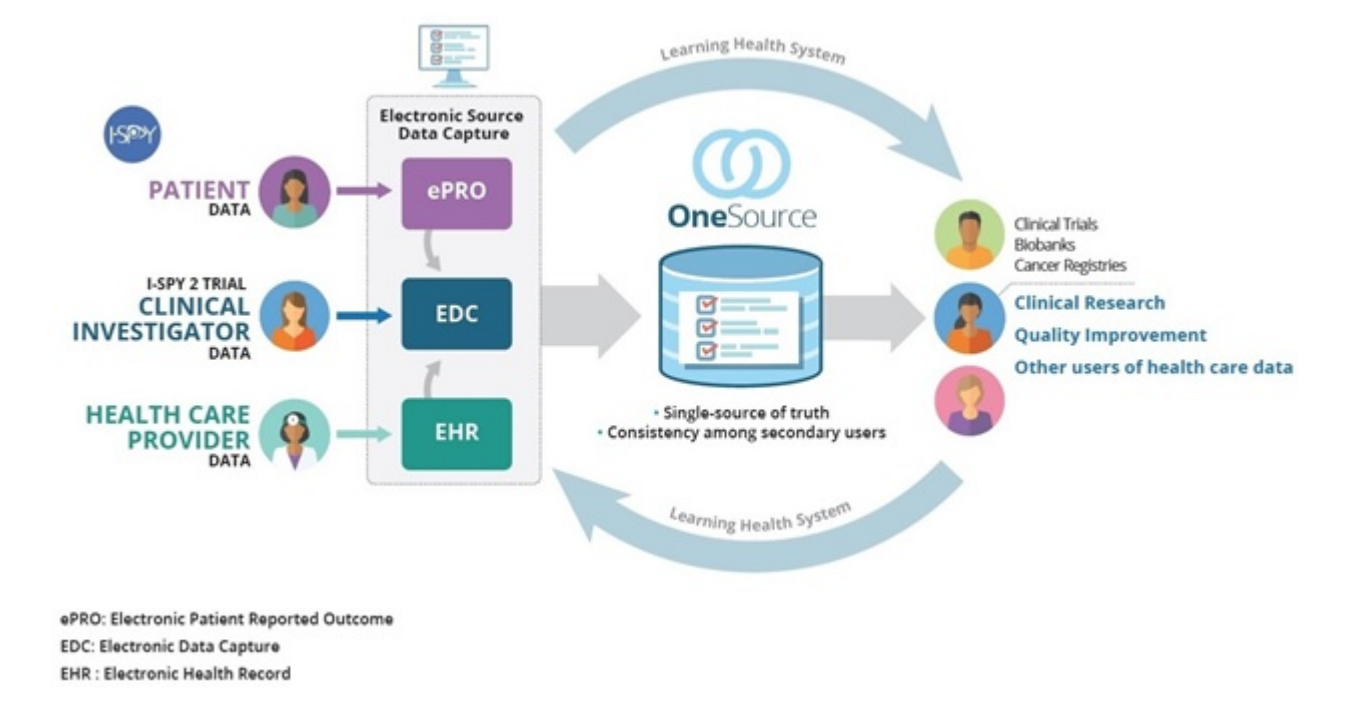
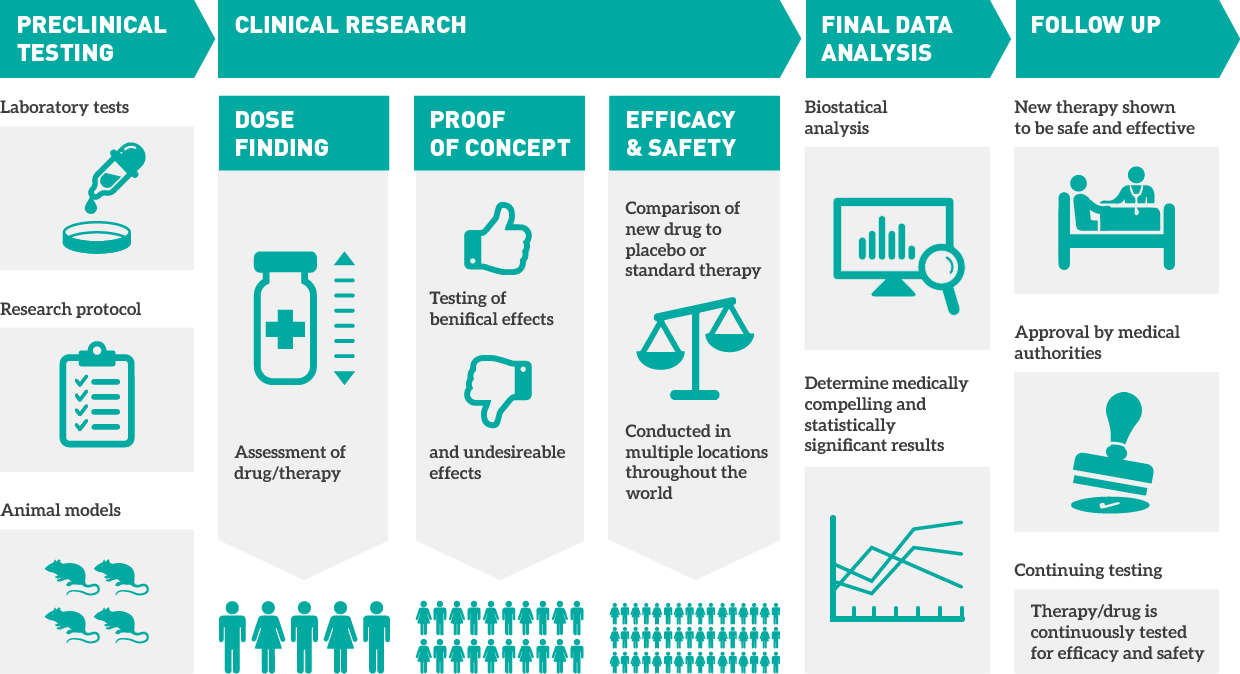
Validation of Electronic Systems to Collect Patient-Reported Outcome (PRO) Data—Recommendations for Clinical Trial Teams: Report of the ISPOR ePRO Systems Validation Good Research Practices Task Force - Value in Health
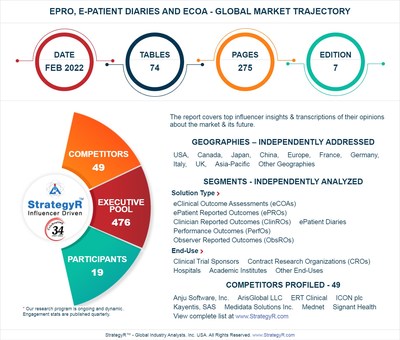
Valued to be $2.8 Billion by 2026, ePRO, E-Patient Diaries and eCOA Slated for Robust Growth Worldwide








![17 Best ePRO Vendors In 2022 & ePRO In Clinical Trials [Ultimate Guide] 17 Best ePRO Vendors In 2022 & ePRO In Clinical Trials [Ultimate Guide]](https://guides.clarahealth.com/content/images/2021/08/AdobeStock_139026638.jpeg)

![How to Use ePRO in Clinical Trials [Complete Guide] How to Use ePRO in Clinical Trials [Complete Guide]](https://www.antidote.me/hubfs/images/licensed/woman-with-phone-095081-edited.jpg)