The obligatory sharing of clinical trial data in the European Union - datenschutz notizen | News-Blog der datenschutz nord Gruppe

EMA's Clinical Trials Information System (CTIS) goes live – EJP RD – European Joint Programme on Rare Diseases

EMA: Points to consider on implications of COVID-19 on methodological aspects of ongoing clinical trials - Meditrial Helpline

Characteristics of Single Pivotal Trials Supporting Regulatory Approvals of Novel Non-orphan, Non-oncology Drugs in the European Union and United States from 2012-2016. - Abstract - Europe PMC

Assessment of the Regulatory Dialogue Between Pharmaceutical Companies and the European Medicines Agency on the Choice of Noninferiority Margins - Clinical Therapeutics
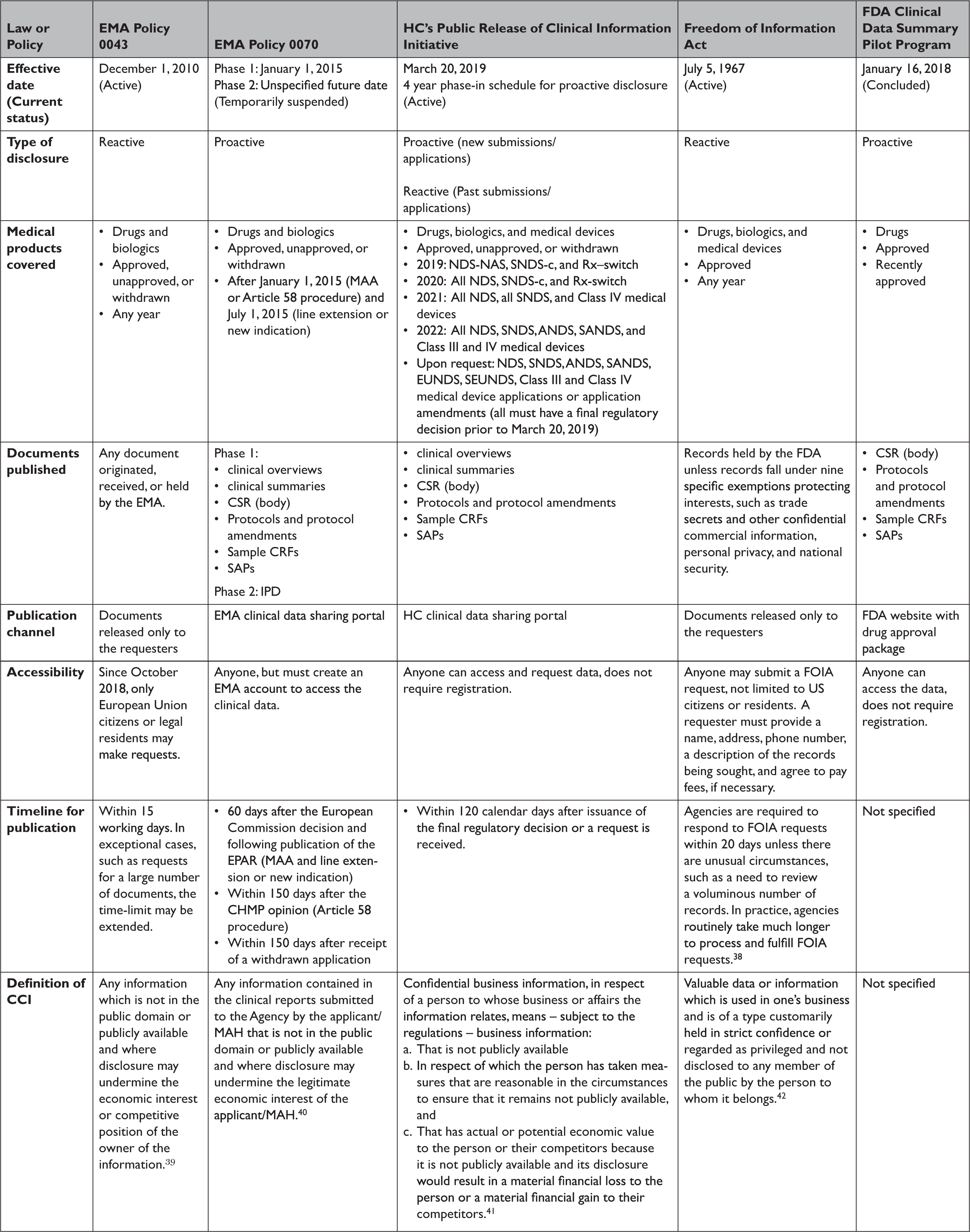
Transparency of Regulatory Data across the European Medicines Agency, Health Canada, and US Food and Drug Administration | Journal of Law, Medicine & Ethics | Cambridge Core

Clinical Trial Regulation Update - PharSafer® - Specialists in Global Clinical and Post Marketing Drug Safety

Comparative overview of Preclinical Data studies and Clinical Trials... | Download Scientific Diagram

Transparency of clinical trials and good governance should be included in the EMA extended mandate | European Alliance for Responsible R&D and Affordable Medicines