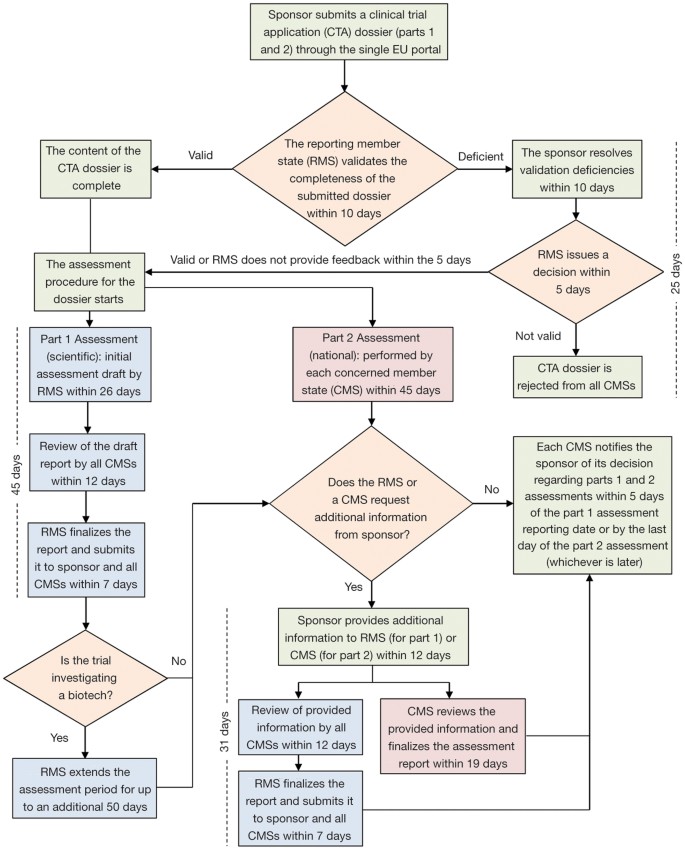
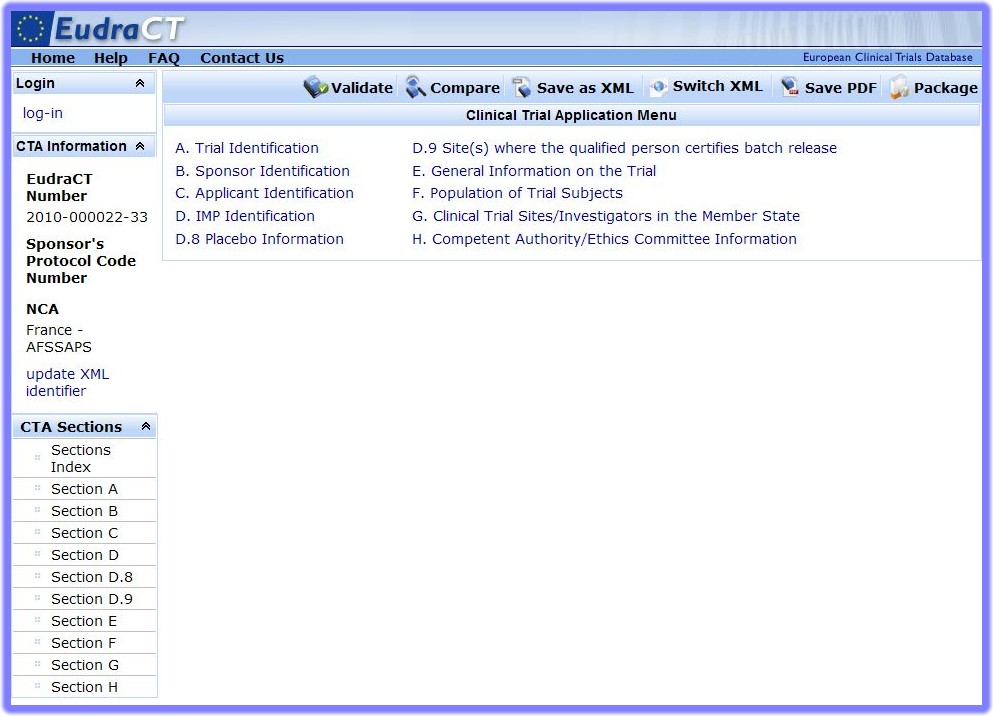
Applying to the Medicines and Healthcare Products Regulatory Agency for a Dentists, Doctors Exemption Certificate (DDX) or a Cl
Declaration of the End of Trial Form (cf. Section 4.2.1 of the Detailed guidance on the request to the competent authorities for
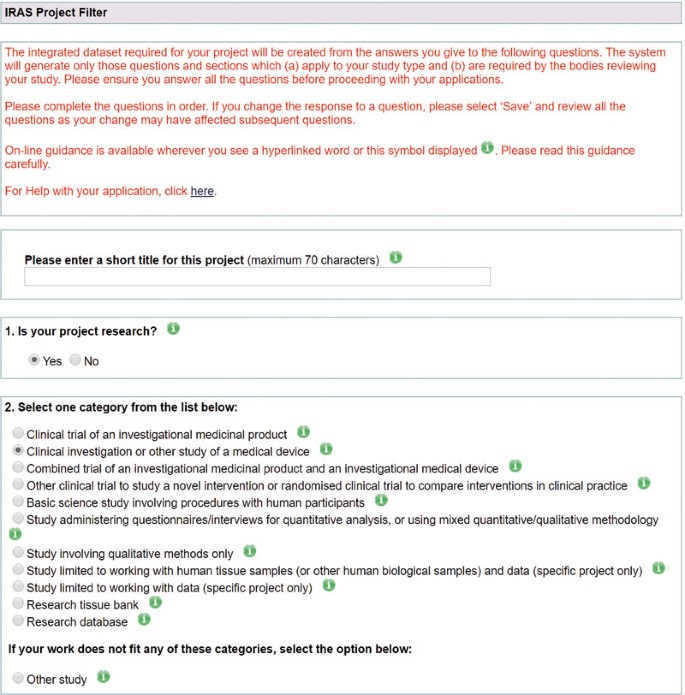
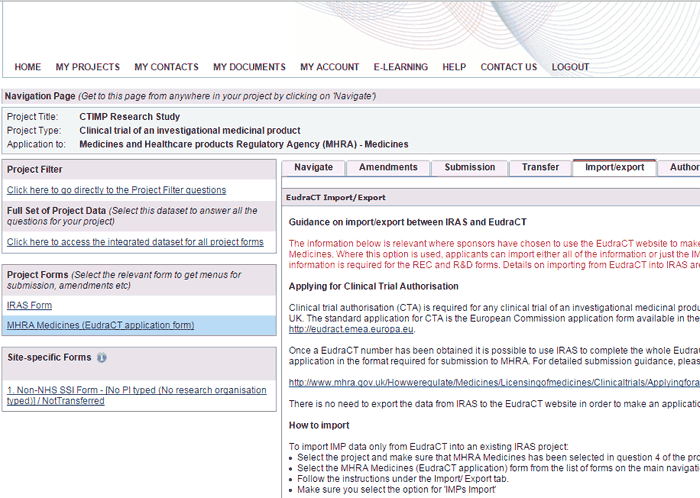
Applying for regulatory approval of a clinical trial of a medical device in the UK – A practical guide | British Dental Journal
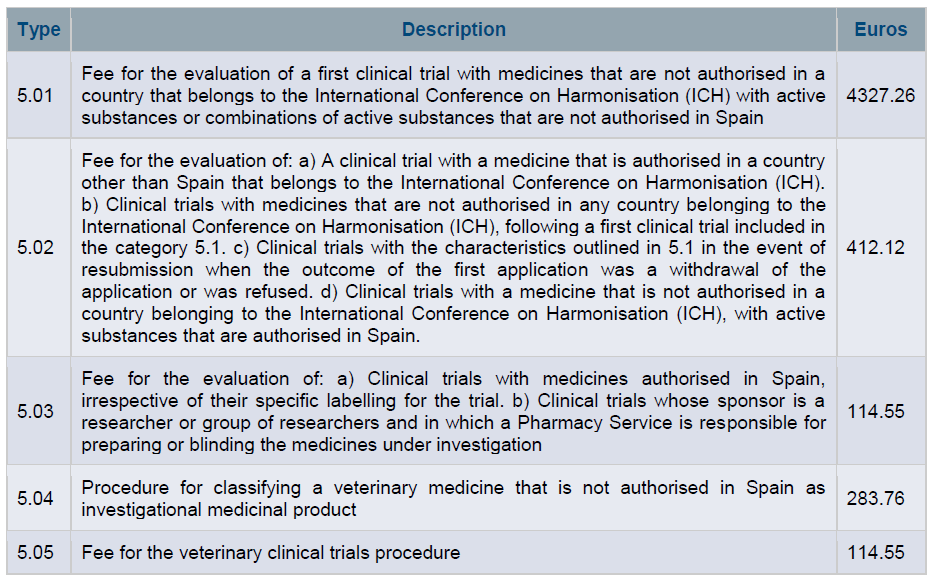
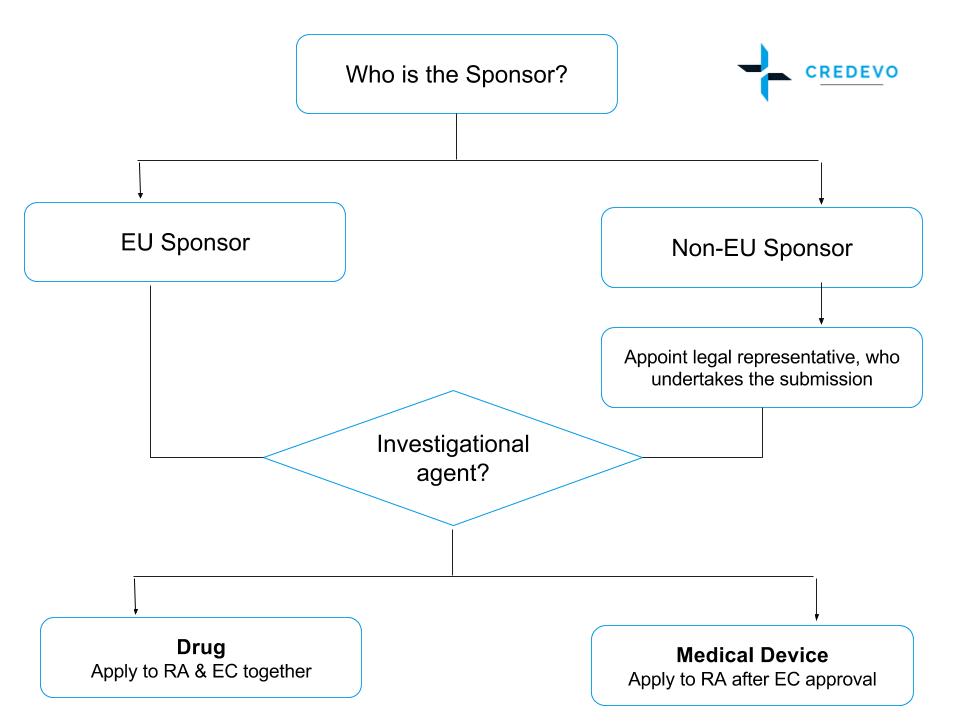
What Are the Documents Required for Clinical Trial Applications to Regulatory Authorities in Europe? - Sofpromed